In Vitro and In Vivo Activity of a Novel Catheter Lock Solution against Bacterial and Fungal Biofilms
Abstract
Central-line-associated bloodstream infections are increasingly recognized to be associated with intraluminal microbial biofilms, and effective measures for the prevention and treatment of bloodstream infections remain lacking. This report evaluates a new commercially developed antimicrobial catheter lock solution (ACL), containing trimethoprim (5 mg/ml), ethanol (25%), and calcium EDTA (Ca-EDTA) (3%), for activity against bacterial and fungal biofilms, using in vitro and in vivo (rabbit) catheter biofilm models. Biofilms were formed by bacterial (seven different species, including vancomycin-resistant Enterococcus [VRE]) or fungal (Candida albicans) species on catheter materials. Biofilm formation was evaluated by quantitative culture (CFU) and scanning electron microscopy (SEM). Treatment with ACL inhibited the growth of adhesion-phase biofilms in vitro after 60 min (VRE) or 15 min (all others), while mature biofilms were completely inhibited after exposure for 2 or 4 h, compared to control. Similar results were observed for drug-resistant bacteria. Compared to the heparinized saline controls, ACL lock therapy significantly reduced the catheter bacterial (3.49 ± 0.75 versus 0.03 ± 0.06 log CFU/catheter; P = 0.016) and fungal (2.48 ± 1.60 versus 0.55 ± 1.19 log CFU/catheter segment; P = 0.013) burdens in the catheterized rabbit model. SEM also demonstrated eradication of bacterial and fungal biofilms in vivo on catheters exposed to ACL, while vigorous biofilms were observed on untreated control catheters. Our results demonstrated that ACL was efficacious against both adhesion-phase and mature biofilms formed by bacteria and fungi in vitro and in vivo.
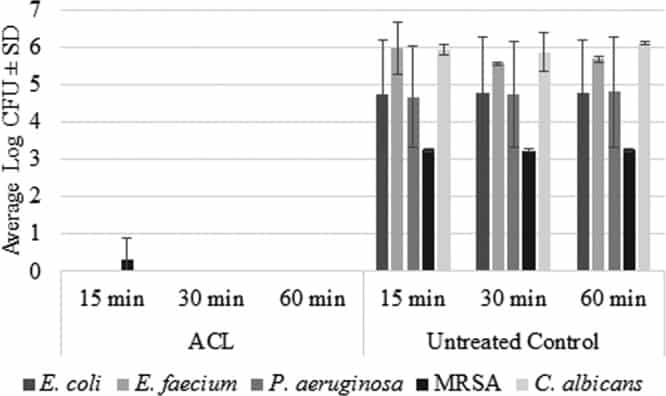
Figure 1
Log CFU values (average ± SD) for ACL-treated disks and untreated control disks against adhesion-phase biofilms formed by TMP-susceptible and TMP/multidrug-resistant E. coli (n = 3), E. faecium (n = 2), P. aeruginosa (n = 3), MRSA (n = 4), and C. albicans (n = 3).
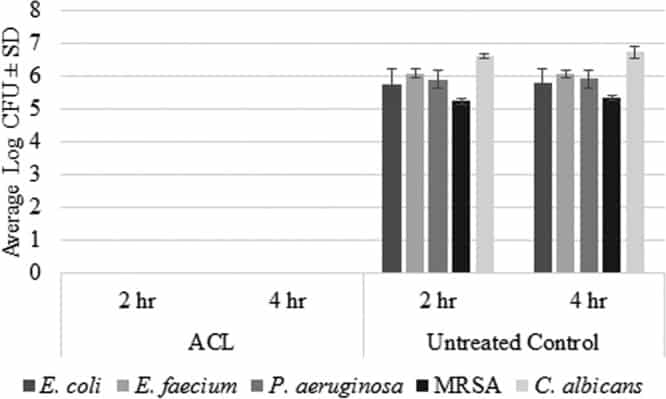
Figure 2
Log CFU values (average ± SD) for ACL-treated disks and untreated control disks against mature biofilms formed by E. coli (n = 3), E. faecium (n = 2), P. aeruginosa (n = 3), MRSA (n = 4), and C. albicans (n = 3).
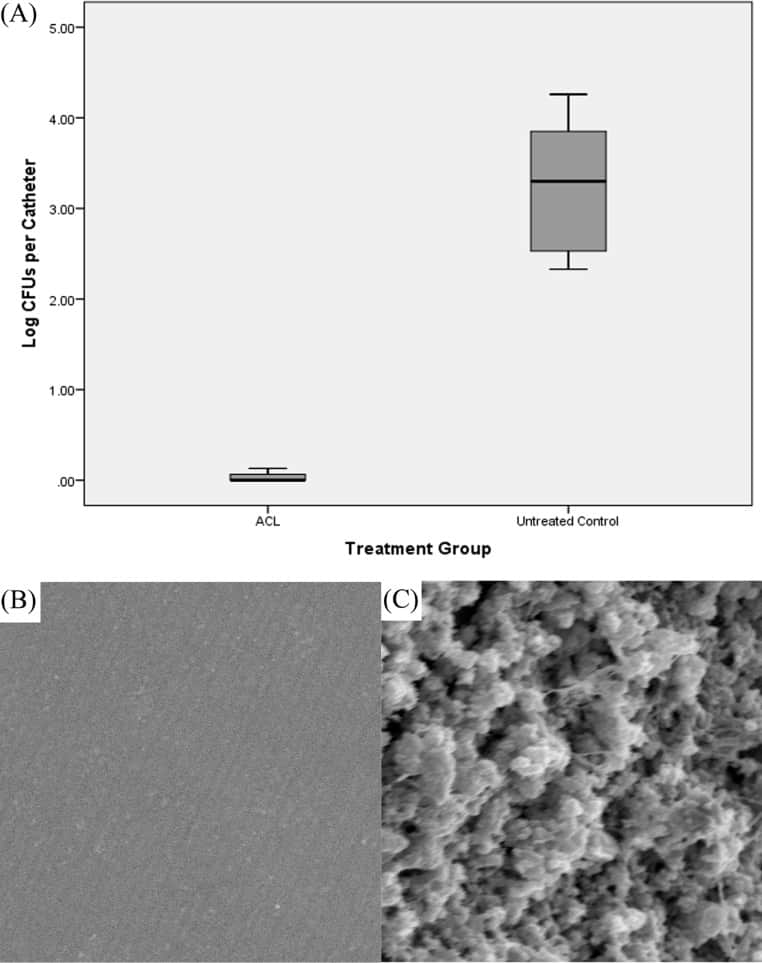
Figure 3
(A) Box plot of bacterial burdens of catheters obtained from ACL-treated animals and untreated control animals inoculated with MRSA biofilms in vivo. The shaded area represents data between the first quartile and the third quartile, the black lines show the median values, and the bars show the minimum and maximum data points. (B and C) Representative scanning electron micrographs of the intraluminal surface of catheters obtained from ACL-treated animals (B) and untreated animals (C). Magnification, ×5,000.
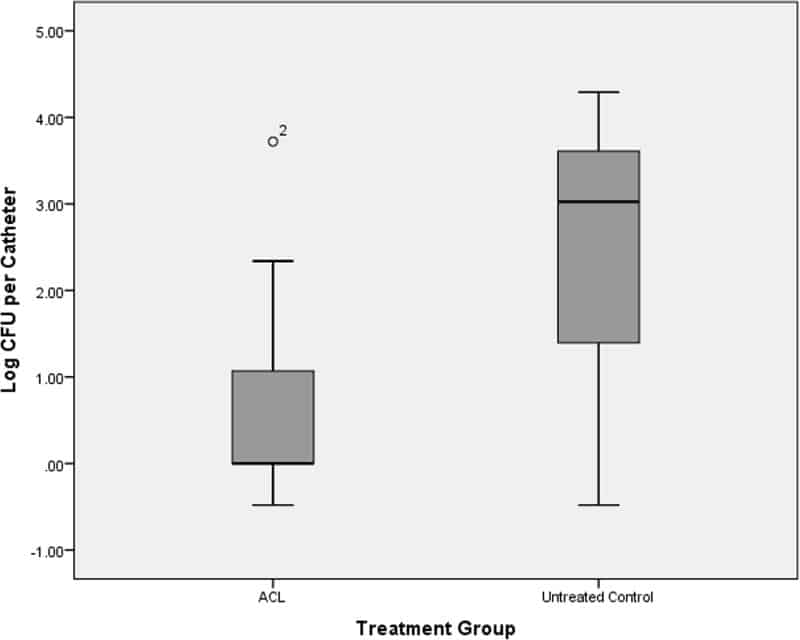
Figure 4
Box plot of fungal burdens of catheters obtained from ACL-treated animals and untreated control animals inoculated with C. albicansbiofilms in vivo. The shaded area represents data between the first quartile and the third quartile, the black lines show the median values, and the bars show the minimum and maximum data points. O2, outlier.