Development of a 96-well Catheter-based Microdilution Method to Test Antifungal Susceptibility of Candida Biofilms
Abstract
Background:
Candida biofilms, which are often associated with device-related infections, including catheter-related bloodstream infections, are resistant to commonly used antifungal agents. Current microtitre (96-well) plate-based methods to determine the antifungal susceptibility of these biofilms do not involve clinically relevant substrates (e.g. catheters), and are not well suited for evaluating the surface topography and three-dimensional architecture of biofilms. We describe a simple, reproducible catheter-based microtitre plate method to form biofilms and evaluate their antifungal susceptibility.
Methods:
Biofilms were formed by Candida species on 5 mm catheter discs placed in microtitre plates and quantified using metabolic conversion of a formazan dye (XTT). The morphology, surface topography and three-dimensional architecture of these biofilms were evaluated by fluorescence, confocal scanning laser and scanning electron microscopy, respectively. The optimized XTT method was used to evaluate the antifungal susceptibility of formed Candida biofilms to fluconazole, voriconazole, itraconazole and anidulafungin.
Results:
Maximum XTT activity was achieved within 90 min. All tested Candida strains formed robust biofilms on catheter discs at both 24 and 48 h (P = 0.66). Biofilms exhibited typical gross morphology, surface topography and architecture, and no difference in biofilm thickness (P = 0.37). The three tested azoles were not active against the biofilms (MIC ≥ 64 mg/L), but anidulafungin possessed potent activity against them (MIC = 0.063-0.125 mg/L).
Conclusions:
The developed method is simple, rapid and reproducible, and requires relatively small amounts of drug. It can be used to perform both high-resolution microscopic analysis of the topography and architecture of biofilms, and evaluation of their antifungal susceptibility.
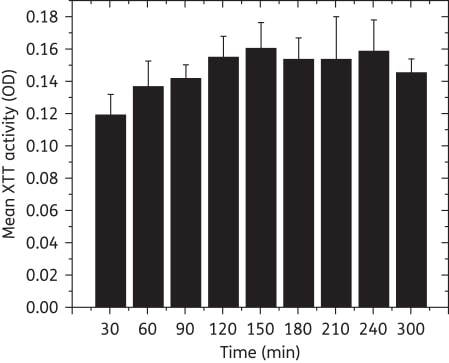
Figure 1
Metabolic activity (XTT OD) of biofilms after incubation for different times. Maximum XTT activity was reached within 90 min, suggesting that the readout time can be reduced from 300 min to 90 min (P = 0.022 versus biofilms grown to 30 min, ≥0.05 for comparisons with biofilms grown to other timepoints). OD, optical density.
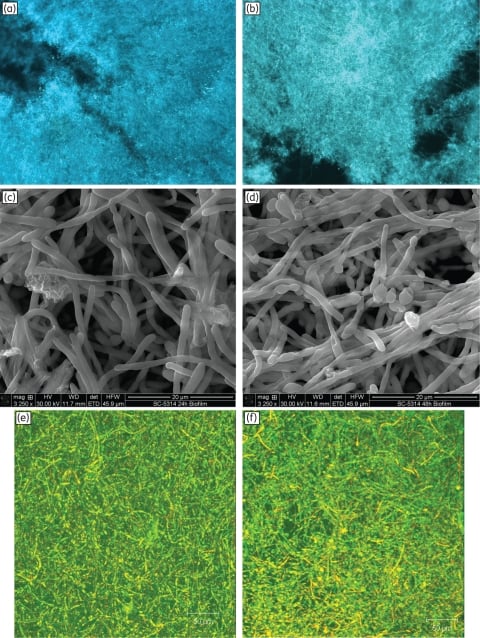
Figure 2
Morphology, surface topography and three-dimensional architecture of Candida biofilms grown to 24 or 48 h. Fluorescence micrographs of biofilms grown to (a) 24 h or (b) 48 h exhibited similar hyphae-rich morphology (magnification: ×10). Scanning electron micrographs of (c) 24 h and (d) 48 h biofilms showed yeast and hyphae, interspersed with granular extracellular matrix (magnification: ×3250). Confocal scanning laser microscopy of (e) 24 h and (f) 48 h biofilms exhibited a highly heterogeneous architecture of mature C. albicans biofilms encased in extracellular material (diffuse green staining; magnification: ×10). Metabolically active cells appear yellow due to co-staining with FUN-1™ (red) and ConA (green) dyes. Biofilms (24 or 48 h) formed on 5 mm SE discs were composed of a dense layer of yeast and hyphal forms, embedded in extracellular polymeric material that had an amorphous granular appearance.