Biofilm Formation by the Fungal Pathogen Candida Albicans: Development, Architecture, and Drug Resistance
Abstract
Biofilms are a protected niche for microorganisms, where they are safe from antibiotic treatment and can create a source of persistent infection. Using two clinically relevant Candida albicans biofilm models formed on bioprosthetic materials, we demonstrated that biofilm formation proceeds through three distinct developmental phases. These growth phases transform adherent blastospores to well-defined cellular communities encased in a polysaccharide matrix. Fluorescence and confocal scanning laser microscopy revealed that C. albicans biofilms have a highly heterogeneous architecture composed of cellular and noncellular elements. In both models, antifungal resistance of biofilm-grown cells increased in conjunction with biofilm formation. The expression of agglutinin-like (ALS) genes, which encode a family of proteins implicated in adhesion to host surfaces, was differentially regulated between planktonic and biofilm-grown cells. The ability of C. albicans to form biofilms contrasts sharply with that of Saccharomyces cerevisiae, which adhered to bioprosthetic surfaces but failed to form a mature biofilm. The studies described here form the basis for investigations into the molecular mechanisms of Candida biofilm biology and antifungal resistance and provide the means to design novel therapies for biofilm-based infections.
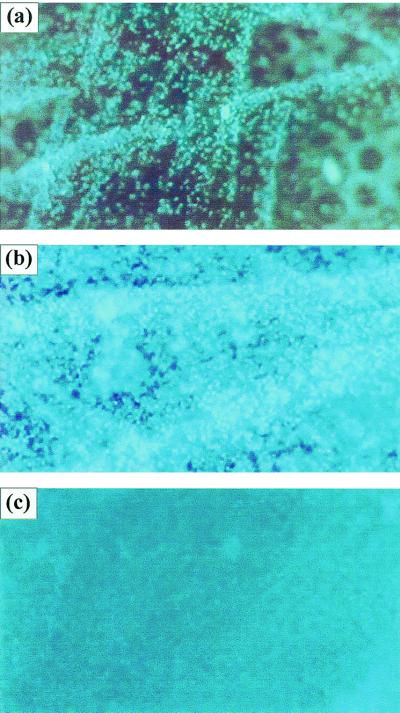
Figure 1
Development of C. albicans biofilm on polymethylmethacrylate strips. Fluorescence microscopy images show the three distinct developmental phases of C. albicans biofilms over a 72-h period: early (a), intermediate (b), and maturation (c) phases. Magnification, ×10.
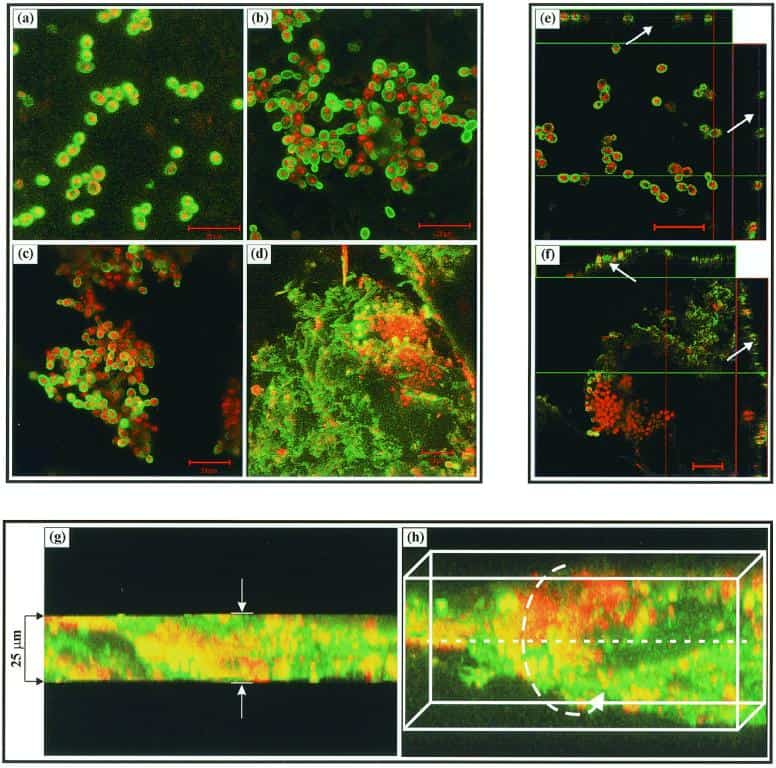
Figure 2
CSLM images of a C. albicans biofilm grown on denture acrylic surface. (a to d) Horizontal (xy) view of reconstructed 3-D images at 0 (a), 8 (b), 11 (c), and 48 (d) h. Bar, 20 μm. (e and f) Orthogonal images of C. albicans biofilms grown to early and maturation phases show that early-phase (0 h) biofilm consisted of mostly yeast cells separated by blank spaces (arrows) (e), while maturation-phase (48 h) biofilm showed metabolically active (red, FUN1-stained) cells embedded in the polysaccharide extracellular material (green, ConA-stained, arrows) (f). (g and h) Thickness of the biofilm (≈25 μm) can be observed in the side view of the reconstruction (g), while a horizontally tilted image (with false 3-D cubes) shows the heterogeneity of the biofilm (h). Magnification, ×40.
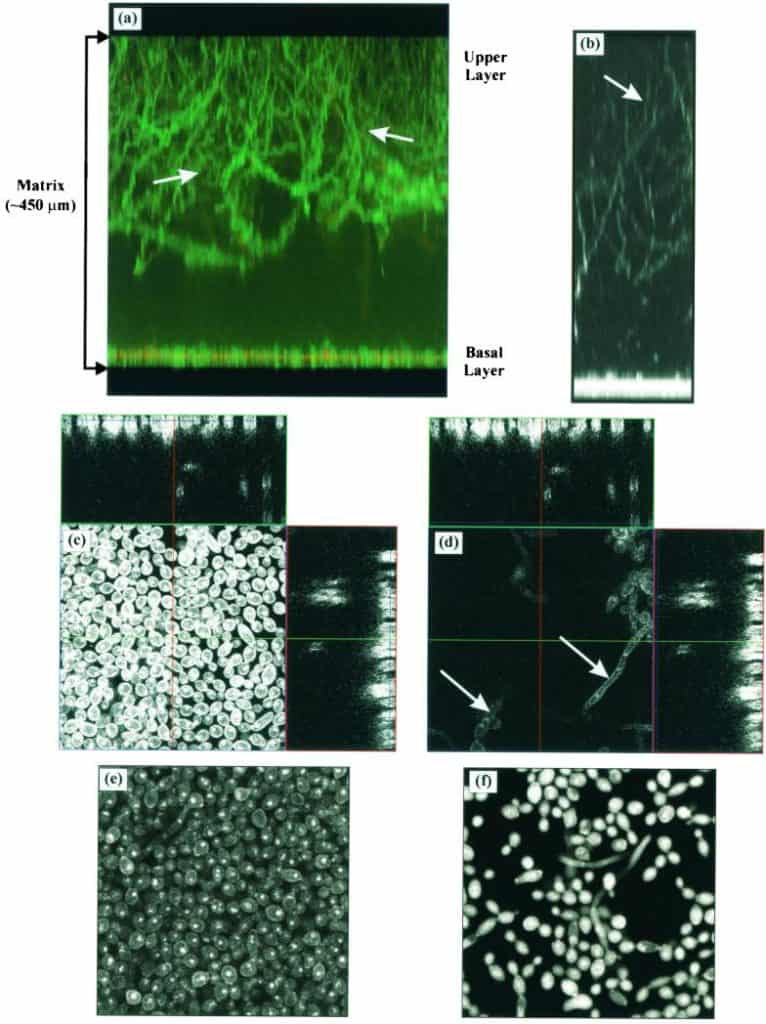
Figure 3
CSLM images of Calcofluor-stained mature C. albicans biofilms formed on silicone elastomer surface. (a and b) Projection (xz or side view) of 3-D reconstructed images showing an approximately 450-μm-thick biofilm with a basal layer (10 to 12 μm thick) consisting of yeast cells and a top layer consisting of hyphal elements (arrow). The extracellular material (ECM) is stained with ConA, resulting in the green color. (c and d) Orthogonal images of the basal (c) and upper layers (d). The ECM-derived haziness seen in mature biofilm (e) is absent when the extracellular material is removed (f). Magnification, ×20.
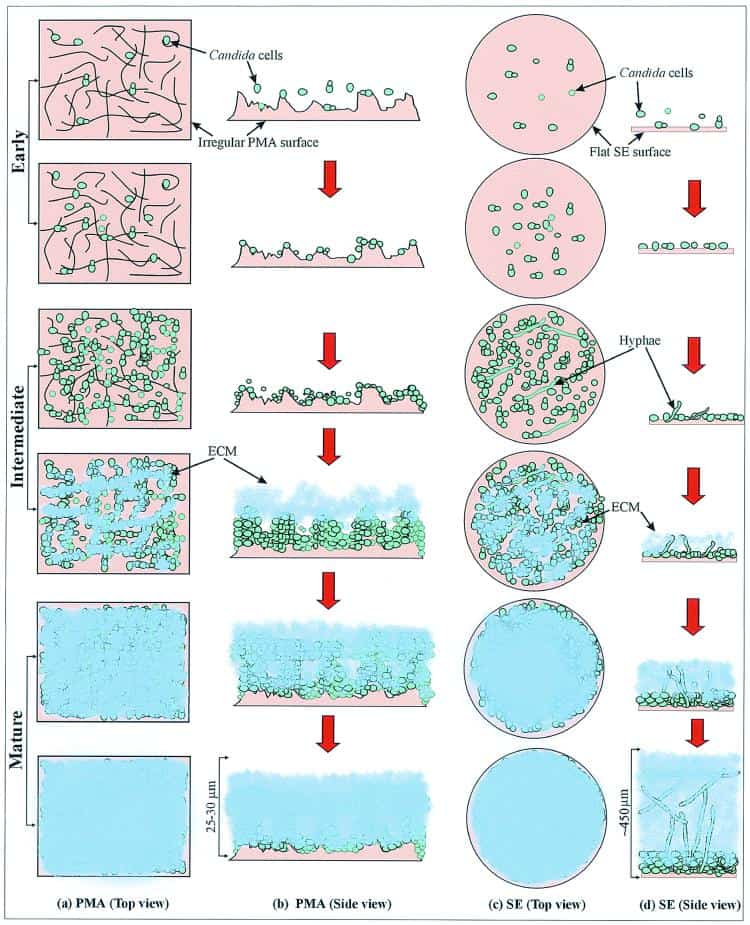
Figure 4
Schematic representation of biofilm development in C. albicans. (a and b) Biofilm grown on polymethylmethacrylate (PMA) strips. (c and d) Biofilm grown on silicone elastomer (SE) disks. Panels a and c represent the substrate seen from the top, while panels c and d show the view from the sides of the PMA strip and SE disk, respectively. ECM, extracellular material.
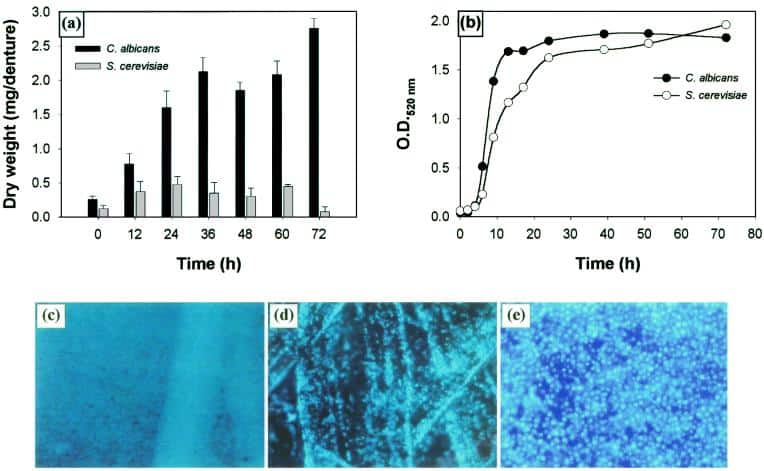
Figure 5
Comparison of the abilities of C. albicans and S. cerevisiae to form biofilms. (a) Quantitative measurement of dry weight of biofilms formed by C. albicans and S. cerevisiae. (b) Growth profiles of planktonic C. albicans and S. cerevisiae. Fluorescence microscopy images of C. albicans (c) and S. cerevisiae (d) grown on polymethylmethacrylate strips. (e) Fluorescence micrograph showing S. cerevisiae growing on silicone elastomer disk (≈3 to 4 μm in thickness). Magnification, ×10.
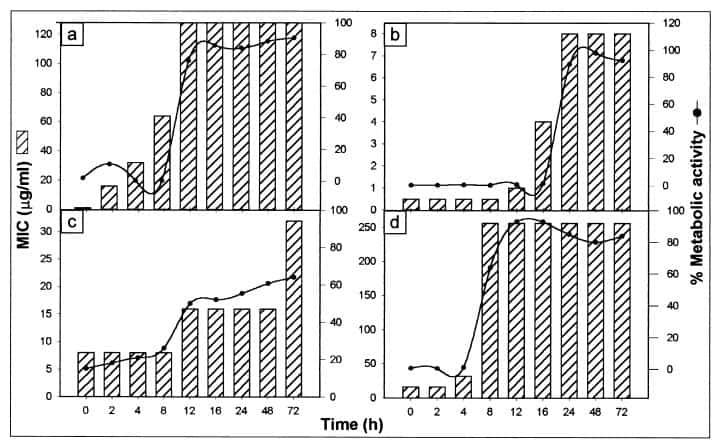
Figure 6
Correlation of biofilm development and metabolic activity with antifungal resistance. The susceptibilities of C. albicans at different stages of biofilm development to fluconazole (a), amphotericin B (b), nystatin (c), and chlorhexidine (d) are represented as histograms. The line curves show percent metabolic activity of growing C. albicans biofilms exposed to fluconazole (64 μg/ml), amphotericin B (4 μg/ml), nystatin (8 μg/ml), or chlorhexidine (64 μg/ml). Metabolic activity was normalized to the control without drugs, which was taken as 100%.
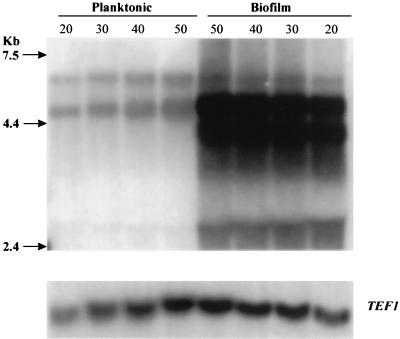
Figure 7
Northern blot analysis of total RNA extracted from planktonic and biofilm-grown C. albicans cells. Total RNA from planktonic and biofilm-grown cells was loaded in various quantities (20, 30, 40, and 50 μg) on a formaldehyde-agarose gel. The resulting Northern blot was probed with a fragment of the C. albicans ALS1 tandem repeats, which hybridized several genes in the family. Probing with a fragment of the TEF1gene served as loading control. Molecular size markers (kb) are shown on the left.