Fusarium and Candida Albicans Biofilms on Soft Contact Lenses: Model Development, Influence of Lens Type, and Susceptibility to Lens Care Solutions
Abstract
Fungal keratitis is commonly caused by Fusarium species and less commonly by Candida species. Recent outbreaks of Fusarium keratitis were associated with contact lens wear and with ReNu with MoistureLoc contact lens care solution, and biofilm formation on contact lens/lens cases was proposed to play a role in this outbreak. However, no in vitro model for contact lens-associated fungal biofilm has been developed. In this study, we developed and characterized in vitro models of biofilm formation on various soft contact lenses using three species of Fusarium and Candida albicans. The contact lenses tested were etafilcon A, galyfilcon A, lotrafilcon A, balafilcon A, alphafilcon A, and polymacon. Our results showed that clinical isolates of Fusarium and C. albicans formed biofilms on all types of lenses tested and that the biofilm architecture varied with the lens type. Moreover, differences in hyphal content and architecture were found between the biofilms formed by these fungi. We also found that two recently isolated keratitis-associated fusaria formed robust biofilms, while the reference ATCC 36031 strain (recommended by the International Organization for Standardization guidelines for testing of disinfectants) failed to form biofilm. Furthermore, using the developed in vitro biofilm model, we showed that phylogenetically diverse planktonic fusaria and Candida were susceptible to MoistureLoc and MultiPlus. However, Fusarium biofilms exhibited reduced susceptibility against these solutions in a species- and time-dependent manner. This in vitro model should provide a better understanding of the biology and pathogenesis of lens-related fungal keratitis.
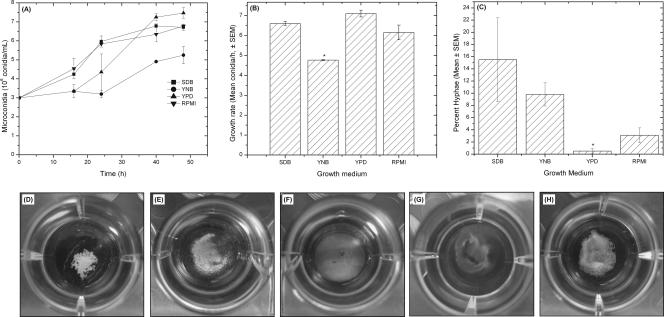
Figure 1
Effects of different culture media on Fusarium growth (A to C) and gross morphology (D to H). Growth curve (A) and growth rate (B) of FSSC 1-b strain MRL8609 in different culture media are shown. (C) Number of hyphal elements present in Fusarium culture after 48 h of growth, expressed as a percentage of the total number of fungal conidia and hyphae {percent hyphae = [number of hyphae/(number of conidia + number of hyphae)] × 100}. (D to H) Biofilms were formed by FSSC 1-b isolate MRL8609 on soft contact lenses, and their gross morphologies were imaged using a digital camera. All lenses tested supported biofilm formation by strain MRL8609. Etafilcon A (D), galyfilcon A (E), lotrafilcon A (F), balafilcon A (G), and alphafilcon A (H) are shown.
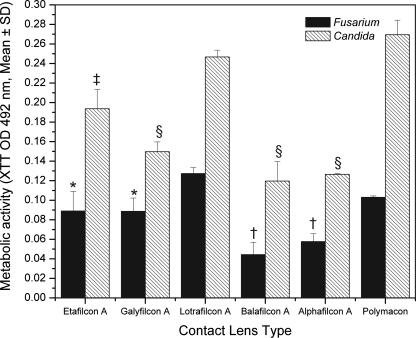
Figure 2
Biofilm formation on soft contact lenses by Fusarium or Candida isolate. Metabolic activities of biofilms formed by FSSC 1-b isolate MRL8609 and C. albicans strain SC5314 on soft contact lenses were determined using the XTT assay as described in Materials and Methods. Experiments were performed in triplicate, and results are expressed as means ± SDs. The asterisk indicates a P value of <0.005 and the dagger indicates a P value of <0.0001 versus results for FSSC 1-b biofilm on a lotrafilcon A lens. The double dagger indicates a Pvalue of <0.005 and the “§” symbol indicates P value of <0.0001 versus results for C. albicans biofilm on lotrafilcon A lens.
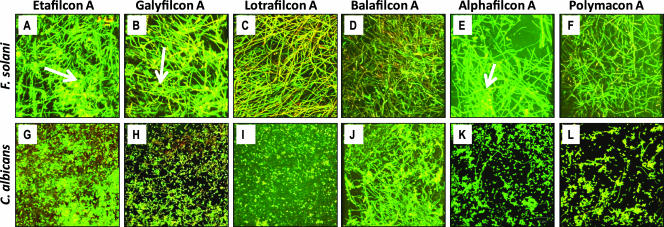
Figure 3
Top-down architecture of Fusarium (A to F) and Candida (G to L) biofilms formed on different soft contact lenses. The FSSC 1-b strain MRL8609 or Candida isolate SC5314 was allowed to form mature biofilms on soft contact lenses and then was stained with ConA and FUN1 dyes. Stained lens-containing biofilms were analyzed by CSLM as described in Materials and Methods. Etafilcon A (A and G), galyfilcon A (B and H), lotrafilcon A (C and I), balafilcon A (D and J), alphafilcon A (E and K), and polymacon lenses (F and L) are shown. Arrows indicate extracellular matrix in the biofilms.
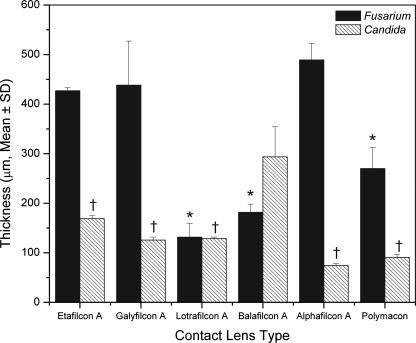
Figure 4
Thicknesses of biofilms formed by FSSC 1-b strain MRL8609 (black bars) or C. albicans strain SC5314 (hatched bars) on soft contact lenses. Strains were allowed to form mature biofilms on soft contact lenses and then were stained with ConA and FUN1 dyes. The stained biofilms were analyzed by CSLM as described in Methods. Biofilm thicknesses were determined by merging all z-stack images into a three-dimensional projection. Side-view images were obtained using CSLM-associated software, and thickness was measured. *, P < 0.0001 versus results for FSSC 1-b strain MRL8609 biofilm formed on alphafilcon A lens; †, P < 0.0001 versus results for C. albicans biofilm formed on balafilcon A lens.
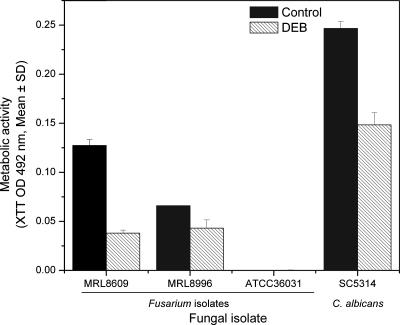
Figure 5
Abilities of three species of Fusarium and Candida albicans to form biofilm on lotrafilcon A lens in the absence (black bars) or presence (hatched bars) of DEB. Biofilms were formed in the absence or presence of DEB on a lotrafilcon A lens as described in Materials and Methods, using the FSSC 2-c ATCC 36031 reference isolate or clinical isolate FSSC 1-b (MRL8609), FOSC 3-a (MRL8996), or C. albicansSC5314. Biofilms were quantified using the XTT metabolic activity assay. Data represent means (± SDs) calculated from three separate experiments.
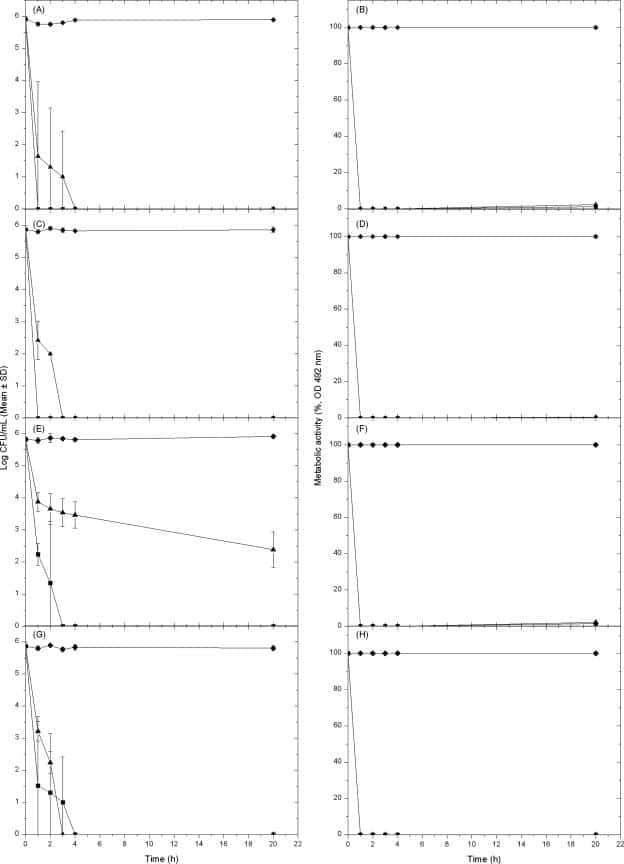
Figure 6
Effect of lens cleaning solutions against three species of Fusarium (A to F) or Candida albicans (G and H) grown planktonically. Susceptibilities of planktonically grown fungal cells were determined using the ISO 14729 methodology (A, C, E, and G) (at 0, 1, 2, 3, 4, or 20 h) or the XTT-based metabolic activity assay with an endpoint criterion of >50% inhibition compared to level for untreated controls (B, D, F, and H). The three species of Fusarium tested were FSSC 1-b (MRL8609) (A and B), FOSC 3-a (MRL8996) (C and D), and FSSC 2-c (ATCC 36031) (E and F), while the C. albicans isolate tested was SC5314. Planktonically grown Fusarium or Candida cells were either untreated (solid diamonds) or treated with MoistureLoc (solid squares) or MultiPlus (solid triangles) solutions. Percent reduction in metabolic activity was calculated for each lens care solution with respect to metabolic activity of biofilm grown in the absence of the disinfectant (which was considered to be 100% activity). Data represent means (± SDs) for three separate experiments.