Effects of a Novel Probiotic Combination on Pathogenic Bacterial-Fungal Polymicrobial Biofilms
Abstract
Dysbiosis of the gut microbiome has been implicated in inflammatory bowel diseases. We have shown that levels of Candida tropicalis, along with those of Escherichia coli and Serratia marcescens, are significantly elevated in Crohn’s disease (CD) patients. Here, we evaluated the ability of a novel probiotic to prevent and treat polymicrobial biofilms (PMB) formed by C. tropicalis with E. coli and S. marcescens Since Candida albicans has been reported to be elevated in CD patients, we investigated the interactions of C. albicans with these bacterial species in biofilm formation. We determined whether the interaction between Candida spp. and bacteria is specific by using Trichosporon inkin and Saccharomyces fibuligera as comparators. Additionally, the effects of probiotics on C. albicans germination and biofilm formation were determined. To determine the ability of the probiotic to prevent or treat mature biofilms, probiotic filtrate was added to the PMB at early (prevention) and mature (treatment) phases. Biofilm thickness and architecture were assessed by confocal scanning laser microscopy. The effects of the probiotic on germination were evaluated in the presence of serum. Exposure of C. tropicalis PMB to probiotic filtrate reduced biofilm matrix, decreased thickness, and inhibited hyphal formation. We showed that C. albicans or C. tropicalis formed significantly thicker PMB than control biofilms, indicating that this interaction is Candida specific. Treatment with probiotic filtrate inhibited C. albicans germination and prevented/treated C. albicans PMB. The designed probiotic may have utility in the management of biofilm-associated gastrointestinal diseases such as Crohn’s and colorectal cancer.IMPORTANCE The effects of diversity of the gut microbiome on inflammation have centered mainly on bacterial flora. Recent research has implicated fungal species and their interactions with other organisms in the inflammatory process. New ways to restore microbial balance in the gut are being explored. Our goal was to identify beneficial probiotic strains that would antagonize these fungal and bacterial pathogens that are elevated in the inflamed gut, and which also have antibiofilm activity. Fungus-bacterium correlation analysis allowed us to identify candidate probiotic species that can antagonize microbial pathogens, which we subsequently incorporated into a novel probiotic formulation. Amylase, which is known to have some antibiofilm activity, was also added to the probiotic mixture. This novel probiotic may have utility for the management of inflammatory bowel diseases by disrupting polymicrobial biofilm formation.
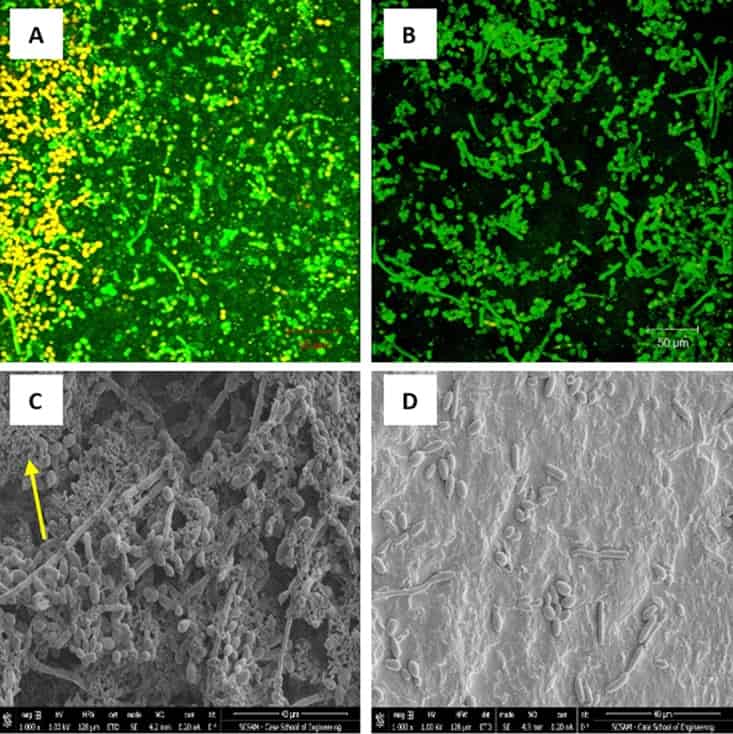
Figure 1
Effect of probiotic on prevention of C. tropicalis, E. coli, and S. marcescens polymicrobial biofilms (PMB). PMB were exposed to probiotic filtrate during the early biofilm phase and allowed to form biofilms. The effects were examined using CSLM and SEM. (A) CSLM micrograph of untreated PMB showing healthy biofilm composed of yeast and hyphal structures. (B) CSLM micrograph of probiotic-treated PMB showing no biofilm matrix and reduced fungal cells. (C) SEM micrograph of untreated PMB showing dense biofilm with yeast and hyphal structures, as well as bacterial aggregates (arrow). (D) SEM micrograph of probiotic-treated PMB showing absence of matrix, very few yeast cells, and no visible bacteria. CSLM magnification, ×100; SEM magnification, ×1,000.
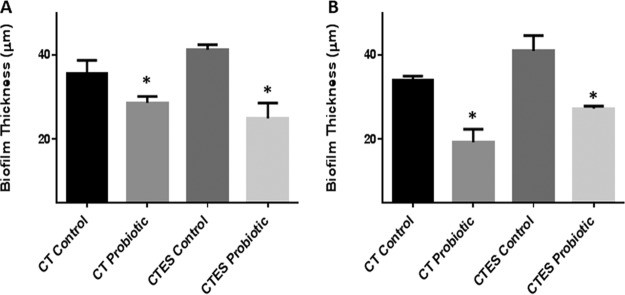
Figure 2
Effect of probiotic on C. tropicalis, E. coli, and S. marcescens (CTES) PMB, as well as biofilms formed by C. tropicalis alone. Vertical (xz) sections or side views of the 3D-reconstructed CSLM images were used to determine biofilm thickness. (A) Prevention of biofilm formation by probiotic. (B) Treatment of biofilms by probiotic. Exposure to probiotic led to a significant reduction in the thickness of PMB and single-species biofilms compared to the untreated controls.
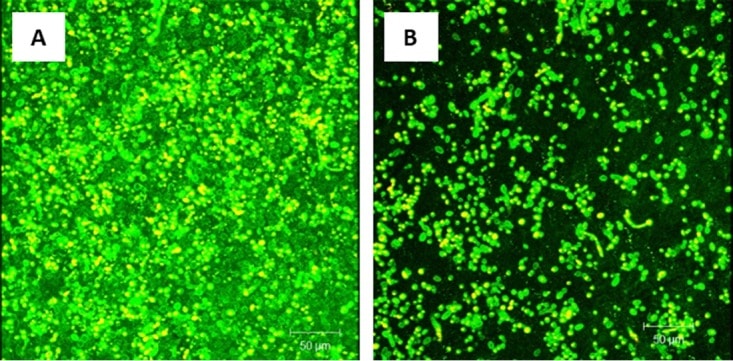
Figure 3
Effect of probiotic on treatment of C. tropicalis, E. coli, and S. marcescens PMB. Mature PMB were exposed to probiotic filtrate and incubated for an additional 24 h. The effects were examined using CSLM. (A) CSLM micrograph of untreated PMB showing healthy biofilm composed of yeast and hyphal structures. (B) CSLM micrograph of probiotic-treated PMB showing no biofilm matrix and reduced fungal cells.
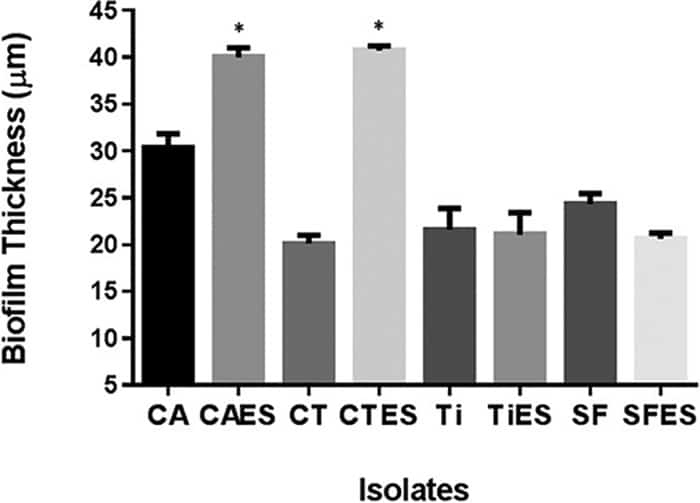
Figure 4
Determination of the specificity of fungus-bacterium interactions in PMB formation. PMB formed by C. tropicalis (CT) or C. albicans (CA) with E. coli (E) and S. marcescens (S) were compared with biofilms formed by T. inkin (Ti) or S. fibuligera (SF) with E. coli and S. marcescens. PMB formed by C. tropicalis or C. albicans were significantly thicker than biofilms formed by C. tropicalis or C. albicansalone. In contrast, no significant difference was seen between PMB formed by T. inkin or S. fibuligera and biofilms formed by these yeasts alone, indicating that the fungus-bacterium interaction in PMB formation is Candida specific.
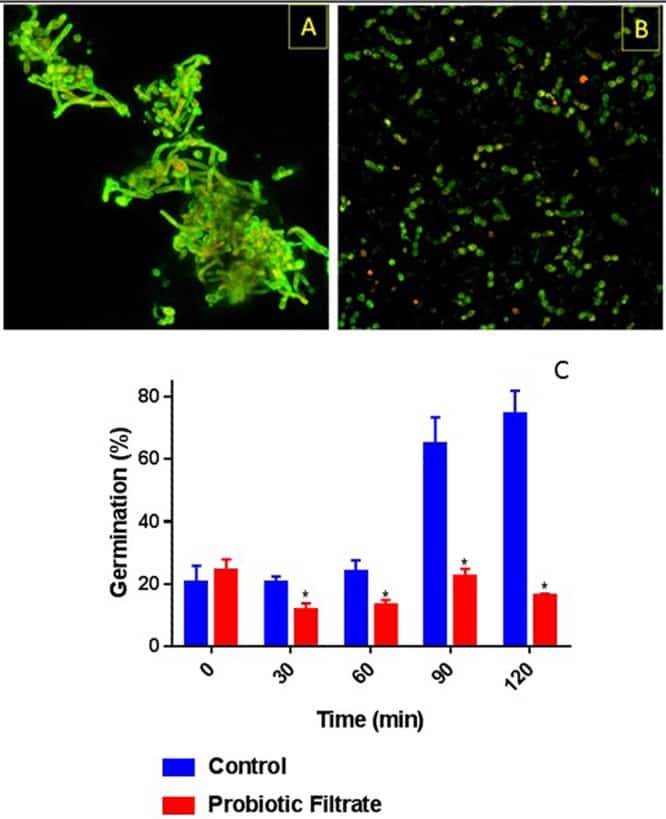
Figure 5
Effect of probiotic on germ tube formation by C. albicans. Ability of C. albicans to form germ tubes was evaluated in the presence or absence (control) of probiotic filtrate. Exposure to probiotic led to inhibition of germ tube formation by C. albicans. (A) CSLM image of control untreated C. albicans showing profuse germ tube formation. (B) CSLM image of probiotic-treated C. albicans showing yeast morphology only with no germ tubes. (C) Quantitative analysis of temporal germ tube formation of probiotic-treated and untreated C. albicans cells showing a significant reduction in percent germination.
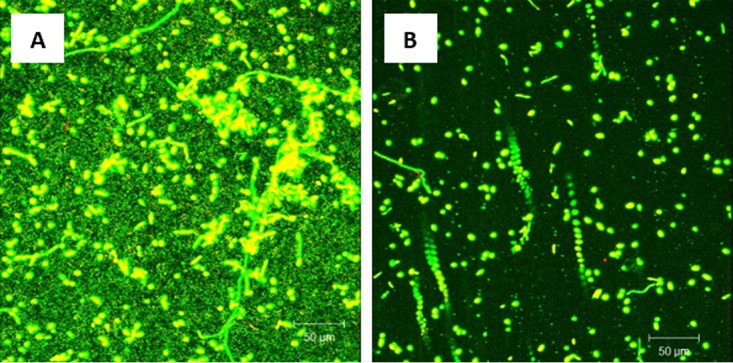
Figure 6
Effect of probiotic on prevention of C. albicans, E. coli, and S. marcescens PMB. PMB were exposed to probiotic filtrate during the early biofilm phase and allowed to form biofilms. The effects were examined using CSLM. (A) CSLM micrograph of untreated PMB showing robust biofilm composed of yeast and hyphae. (B) CSLM micrograph of probiotic-treated PMB showing inhibition of biofilm with absence of extracellular matrix and reduced fungal elements. Magnification, ×100.
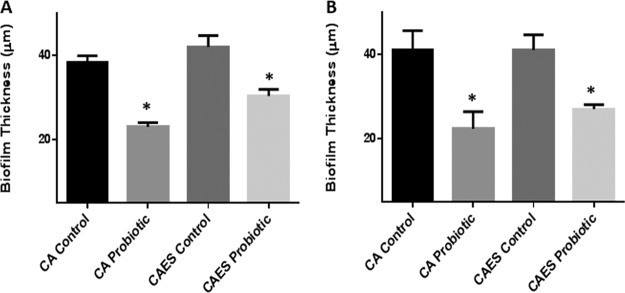
Figure 7
Effect of probiotic on C. albicans, E. coli, and S. marcescens PMB, as well as biofilms formed by C. albicans alone. Vertical (xz) sections or side views of the 3D-reconstructed CSLM images were used to determine biofilm thickness. (A) Prevention of biofilm formation by probiotic. (B) Treatment of biofilms by probiotic. Exposure to probiotic led to a significant reduction in the thickness of PMB and single-species biofilms compared to that in untreated controls.
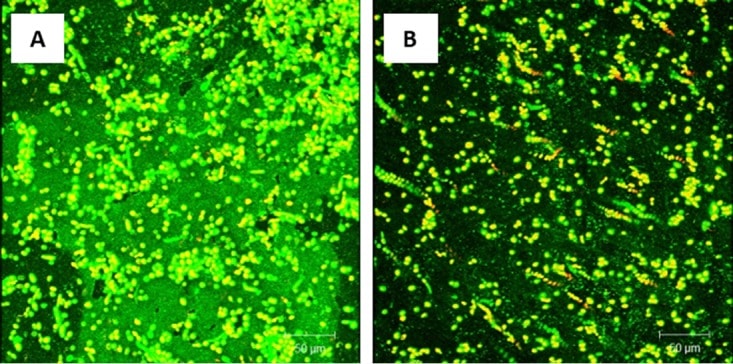
Figure 8
Effect of probiotic on treatment of C. albicans, E. coli, and S. marcescens PMB. Mature PMB were exposed to probiotic filtrate and incubated for an additional 24 h. The effects were examined using CSLM. (A) CSLM micrograph of untreated PMB showing enriched biofilm extracellular matrix. (B) CSLM micrograph of probiotic-treated PMB showing absence of biofilm matrix and reduced fungal cells.