A Rabbit Model for Evaluation of Catheter-associated Fungal Biofilms
Abstract
Most cases of catheter-related bloodstream infections (CRBSIs) involve colonization of micro-organisms on catheter surfaces where they eventually become embedded in a biofilm. Fungal biofilm formation is studied using a number of techniques, involving the use of a wide variety of substrates and growth conditions. In vitro techniques involving use of confocal scanning laser/scanning electron microscopy, metabolic activity assay, dry weight measurements and antifungal susceptibility assays are increasingly used by investigators to quantify and evaluate biofilm morphology. However, there are not many in vivo models used to validate biofilm-associated infections. In this protocol, we describe clinically relevant rabbit model of C. albicans biofilm-associated catheter infection to evaluate the morphology, topography, and architecture of fungal biofilms. The methods described here can be completed in a typical laboratory setting. Evaluation of the formation of fungal biofilms on catheters in vivo, their analysis using scanning electron microscopy (SEM) and quantitative catheter culture (QCC) and treatment of biofilms using antimicrobial lock therapy can be completed using the described methods in ~20-25 days. This model has utility in evaluating the efficacy of lock solutions. In addition, it is a useful approach for characterizing/comparing the formation of biofilms by wild type and isogenic mutants including clinical isolates in vivo. This model can also be used for testing different biomaterials.
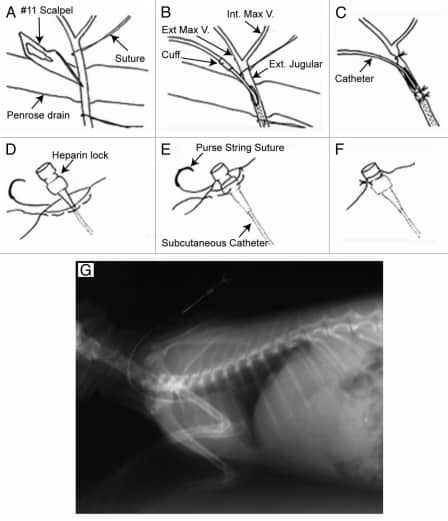
Figure 1
Surgical placement of the intravenous catheter. (A–C) Catheter insertion into the external jugular vein; (D–F) attachment of the heparin lock device to skin (G) postoperative venogram of catheter placement. Adapted from Schinabeck.8

Figure 2
Mature in vivo C. albicans biofilm formation during rabbit model development. Scanning electron micrographs of C. albicans biofilms adherent to the intraluminal surface of catheters showing biofilm architecture at 7 d post-infection (A) magnification, 1,500x (B) magnification, 6,500x. There was no difference in biofilms at 3 d post-infection (data not shown). Adapted from Schinabeck et al.8
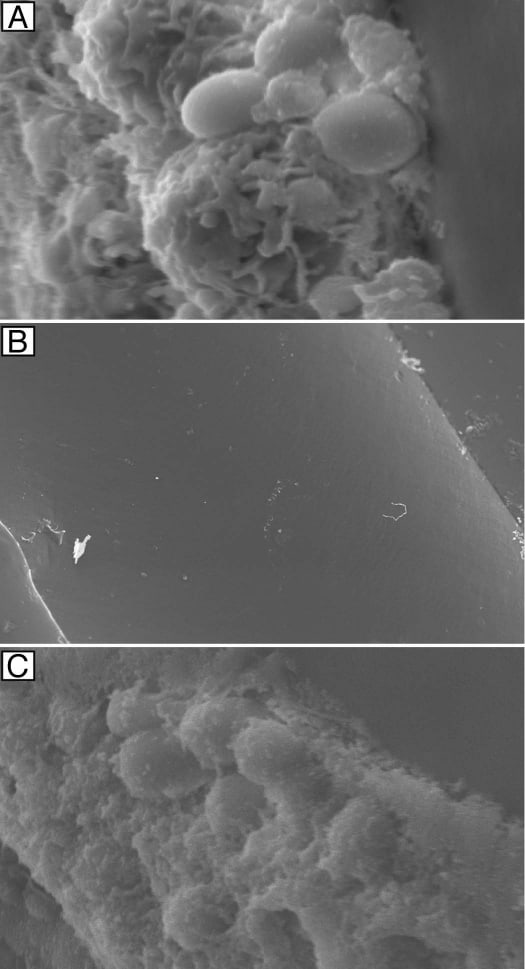
Figure 3
Effectiveness of antifungal lock therapy. Scanning electron micrographs of intraluminal catheter surfaces following 7 d of therapy with heparinized saline (magnification, 5,000x) (A), liposomal amphotericin B (magnification, 121x) (B) and fluconazole (magnification, 3,500x) (C) are shown. Adapted from Schinabeck et al.8